
Updated 10.03.2024
“In science and medicine, information is constantly changing and may become out-of-date as new data becomes available. All articles, videos and interviews were up to date at the time of publication. If you are a patient, this information is not intended to replace advice you may be given by your medical team. If using our Accessibility Tool note that it uses Google Translate which may not always accurately represent the original text.”
If CLL is suspected, a number of tests will be carried out.
FBC is one of the key tests and is usually the first step.
Blood contains three types of cells:
For an FBC a sample of blood is taken and examined in the laboratory. The number and appearance of red cells, white cells and platelets are recorded and compared with normal blood test results.
However, an FBC on its own does not confirm CLL and further tests will be required.
The most common tests include:
A lymph node biopsy is a minor surgical procedure where a small sample is taken from a lymph node, then examined under a microscope to help confirm the diagnosis.
Further tests are carried out when the time comes to consider treatment.
Once diagnosed, your consultant will ‘stage’ your CLL. Staging is used to describe where the CLL is located and the extent to which the CLL is affecting the blood count and the number and size of lymph nodes. Staging CLL in this way helps your consultant to predict how quickly the cancer may grow and to keep track of it.
There are two main systems to stage CLL. Most doctors in the UK use the Binet system. In the USA, doctors commonly use the Rai system.
The Binet Staging System
This is a three-step staging system (A, B and C) based on the number of swollen lymph node areas and blood cell counts.
Stage A
Stage B
Stage C
It is possible to have CLL and still have a good quality of life.
Many patients have no symptoms and carry on with life as normal. For some, their CLL may be so slow developing that treatment is not necessary. Others can manage their condition with the appropriate treatment.
New treatments introduced in the last few years mean that, generally speaking, patients now have a better prognosis (outlook) than before.
There are also things you can do to help yourself:
As a CLL patient, you will need to have regular blood tests to monitor the level of CLL cells in your blood and also to look at your general health.
You will find it useful to keep a copy of your blood results to help you see how you are progressing.
One of the main tests your medical team is interested in is the Full Blood Count (FBC). This measures how many blood cells of each kind there are in your blood. The FBC is important as it shows how the CLL, or treatment, is affecting you. The following chart shows the main blood counts that your medical team will be focusing on. It is useful to know some of the medical terms that may be used in discussions with your medical team.
| White Cells | Red Cells | Platelets | |
| Medical name: | Neutrophils | Erythrocytes | Platelets |
| Lymphocytes | Thrombocytes | ||
| What they do: | Fight infection | Carry oxygen | Stop bleeding |
| Low counts are called: | Neutropenia | Anaemia | Thrombocytopenia |
| Symptoms | Infections | Tiredness, Breathlessness | Bruising, bleeding |
In a healthy person, the blood counts usually stay the same, with slight variations (both up and down) over time or because of infections. The table below shows how many of each kind of blood cell a healthy person will have. If your counts are outside the ranges shown, your doctor will monitor you carefully to see if there is a trend that could in the future lead to treatment.
| White cells | Red cells | Haemoglobin* | Neutrophil | Platelets | |
| (109/1) | (109/1) | (g/dl) | (109/1) | (109/1) | |
| Adult Male | 4.0 to 11.0 | 4.5 to 6.5 | 13.0 to 18.0 | 2.0 to 7.5 | 150 to 440 |
| Adult Female | 4.0 to 11.0 | 3.8 to 5.0 | 11.5 to 16.5 | 2.0 to 7.5 | 150 to 440 |
Note that these counts must be assessed by a doctor. They will be different for patients of African-Caribbean decent.
*Haemoglobin is a protein used by red blood cells to distribute oxygen to other tissues and cells in the body.
With CLL, total white cell counts tends to go up, often well above normal, while the other cell counts tend to go down. This is because the white cells crowd out the other cells or prevent from them being manufactured. However, remember:
Rule of thumb
As a general guide, it is important to be aware that the white count is most useful at the time of diagnosis to identify CLL. However, after diagnosis, changes in the CLL cells (i.e. the white cells increasing) are not as useful in monitoring CLL as changes in the normal cells (i.e. falling haemoglobin and/or platelets.)
Normally, your immune system helps your body to fight infections. However, in CLL patients, the immune system can be weakened, and there is an increased risk of more severe infections. Your doctor or nurse may want to talk to you about this, and it will help you to understand some of the terms that might be used.
Your immune system is a network of cells, tissues and organs, which protect your body against infection. Lymphocytes play an important part in this. There are lots of different types of lymphocyte, but the important ones to know about are T cells and B cells. These are types of white blood cells which fight infection. CLL affects B cells by allowing them to grow out of control, because they don’t ‘switch off’ and die when they should. They then crowd out other cells, like T cells, from the bone marrow. They may swamp your lymph nodes and spleen, making them enlarged. When this happens, your body can’t produce enough T cells, and the B cells that are produced are immature and don’t work properly.
One of the key measures of how your CLL may be progressing is to look at the number of white cells in your blood. This is usually done by examining the Absolute Lymphocyte Count (ALC), and you may hear this mentioned by your consultant.
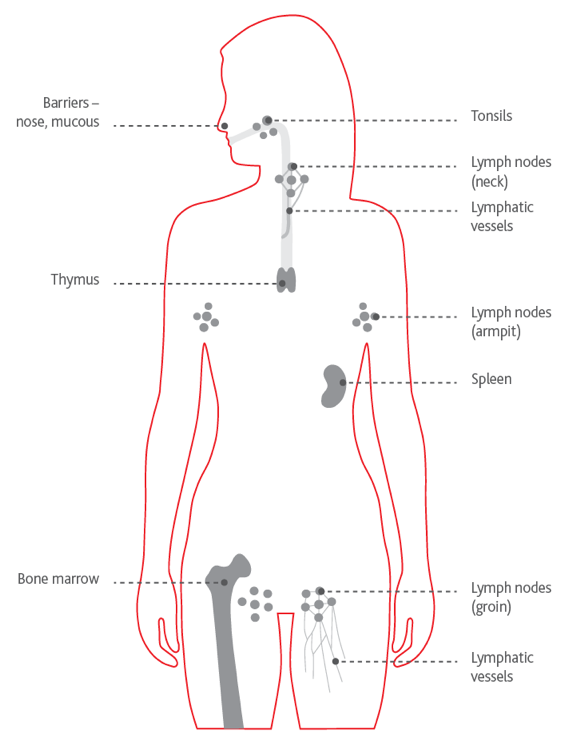
Your lymphatic system
The lymphatic system is a network of thin tubes called lymph vessels, that run around your body. Connected to the vessels are lymph nodes or lymph glands. In CLL, these can become swollen as the abnormal lymphocytes build up there. The ones in your neck, armpits and groin are usually most affected and you may also experience swelling of your spleen.

If you have CLL, you are more likely to pick up infections. This is because CLL weakens your immune system. It is important, therefore, to make sure you are vaccinated for a range of infections as soon after diagnosis as possible. You may also need regular booster vaccinations. Your doctor will advise you about this.
You must NOT receive vaccines which contain live or attenuated (weakened) viruses. These include: yellow fever, oral polio, measles, smallpox, MMR and shingles.
Also note that babies who have received the oral polio vaccine should be avoided for at least a week, as they can pass on the live virus. Similarly, avoid children who have been recently vaccinated using a live virus including the nasal flu vaccine.
Your healthcare team will advise you, but the most useful vaccinations you should consider are:
Flu Vaccine. This may not work for you as well as for people without CLL, but it should offer at least some protection. You should have this vaccine annually. Your close family should be vaccinated too as this will protect them from getting flu and from passing it on to you.
Pneumonia. For pneumococcus, modern practice for CLL patients is to give two vaccines some time apart. These are known as Prevnar-13 (child vaccine) and the usual Pneumovax-23. You should talk to your CLL consultant about having these. Your G.P. may not be aware of this. It is not necessary to have this vaccine annually. Your doctor will advise when you may need to renew it.
Shingles. The only vaccination currently available for people with blood cancer is the non-live vaccine Shingrix. Until September 2023 it can be given only to people aged 70 – 79, but from September it will be available from age 50, with no upper limit. You can find more about shingles here.
For more information on vaccinations for CLL patients, see the video of the presentation given by Dr Helen Parry at the CLLS Birmingham Conference in 2018 here.
If you are getting lots of infections and your antibodies (infection-fighting proteins in your blood) are low, you may be offered IVIg as an infusion every 7 days or so to help boost your immune system. Immunoglobulin can also be offered to some patients as self-administered injections under the skin at home. This is usually given every 1 to 2 weeks can also be offered to some patients as self-administered injections to be done at home.
If you become significantly anaemic, either because of your CLL or during treatment, you may require one or more blood transfusions. If you’ve been treated with certain chemotherapy drugs, such as fludarabine, bendamustine and alemtuzumab (Campath) and need a transfusion, you must receive irradiated blood products. Note that this applies to platelet transfusions also. The transfusion of non-irradiated blood is not a problem for CLL patients who haven’t had these drugs.
In addition, patients undergoing peripheral blood lymphocyte collections for future CAR-T cell re-infusion should receive irradiated cellular blood components for 7 days prior to and during the harvest, to prevent the collection of viable allogeneic (donor) T lymphocytes. Irradiated blood components should continue to be used until 3 months following CAR-T cell infusion unless conditioning, disease or previous treatment determine indefinite duration, for example, previous purine analogue treatment (Fludarabine or Bendamustine).’
For this reason, if you fall into this category, it is advisable to wear a wristband identifying you as an at-risk leukaemia patient who needs irradiated blood products. Your consultant or Clinical Nurse Specialist should give you a warning card that you should always carry with you.
For CLL patients infection can be a real risk. However, you can reduce this by:
Of course, there is a balance to be found between trying to live a normal life and taking precautions, and each person will decide for themselves what level they are prepared to take.
Seek advice from your consultant or GP regarding a prescription for a course of antibiotics for you to have to hand in the event of getting an infection.
Who we are
We are a patient led UK charity, our mission is to support and empower CLL patients, their families and their carers.
Read moreOur 24 hour membership telephone number is: 0800 977 4396
About CLL
CLL is a malignancy of B lymphocytes, one of several types of cells of the immune system.
Read moreAbout CLL
Donate
Donate to support our CLL community
Donate nowHealthUnlocked
Connect to others who understand
Open in a new tab